The Wyatt Laboratory
Research
Description of Research Program
A daunting task in the development of a broadly effective HIV-1 vaccine is to elicit humoral immune responses directed toward conserved, functional elements of the HIV-1 exterior envelope glycoprotein, gp120. Two functions common to virtually all HIV-1 gp120 glycoproteins are the ability to bind the primary virus receptor, CD4, and to bind to the more recently defined co-receptors. In a collaborative study, we have recently solved the crystal structure of gp120, in complex with CD4 and a neutralizing antibody. This structure uniquely provides us with insight regarding the means HIV-1 employs to mask these essential functional elements.
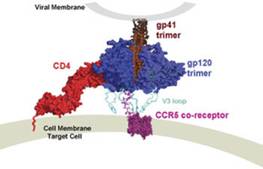
HIV-1 is tropic for CD4-positive cells by virtue of the high affinity interaction between the HIV-1 gp120 glycoprotein, and CD4, which acts as the primary virus receptor. The gp120 molecule is derived from a gp160 precursor glycoprotein and noncovalently associates with the transmembrane glycoprotein, gp41, to form trimeric complexes on the virus or cell surface. The mature HIV-1 gp120 is comprised of five regions conserved among viral strains (C1-C5) and five regions that exhibit considerable strain-to-strain variability (V1-V5). Besides CD4, HIV-1 requires co-factors to achieve entry into target cells. The second receptors used by HIV-1, primarily CXCR4 and CCR5, belong to a family of seven-membrane spanning, G-linked proteins which normally function as chemokine receptors.
|
|
|
A structure-based schematic representation of the HIV-1 envelope glycoprotein trimer interacting with the primary receptor, CD4, and the co-receptor, CCR5 present on the target cell. The gp120 exterior envelope glycoprotein is modeled as a trimer in blue in non-covalent association with the gp41 trimeric transmembrane glycoprotein in brown. The protruding gp120 third major variable loop (V3) is shown in cyan. |
During the course of HIV-1 infection, neutralizing antibodies are elicited against various elements of gp120. Neutralizing antibodies appear to be an important component of the host immune response. Most clinical (primary) HIV-1 isolates are relatively resistant to neutralizing antibodies, suggesting that these viruses are selected by the presence of neutralizing antibodies in infected humans. In many HIV-1 infected individuals, two classes of neutralizing antibodies are elicited: strain-restricted and broadly cross-reactive antibodies. The strain-restricted antibodies appear early after infection and are generally directed against linear determinants within the gp120 third variable region. This class of antibodies has been relatively easy to generate in both primate and non-primate animal systems but is not broadly protective. A subset of the broadly neutralizing antibodies recognizes conformationally dependent, discontinuous epitopes that overlap with the discontinuous CD4 binding site on gp120 and are termed CD4 binding site antibodies. A second limited subset of broadly neutralizing antibodies recognizes discontinuous gp120 epitopes, overlapping with the chemokine receptor binding site, that are better exposed upon CD4 binding and thus are referred to as CD4-induced antibodies. In naive individuals, the presence of broadly cross-reactive neutralizing antibodies and strain-restricted antibodies might help prevent or limit HIV-1 infection following exposure to the virus.
The broadly neutralizing antibodies, however, are difficult to elicit in animals using wild-type gp120 glycoproteins as an immunogen. Through several hypotheses based on evolving structural information of HIV-1 envelope glycoproteins, we will determine if rational modification of gp120 glycoproteins will influence the exposure and presentation of conserved, functional structures to the immune system. The elicitation of antibodies with an expanded breadth of neutralization capacity may thereby be enhanced. This rational approach to improve gp120 HIV-1 envelope glycoprotein immunogenicity may have a significant impact on HIV-1 vaccine design.