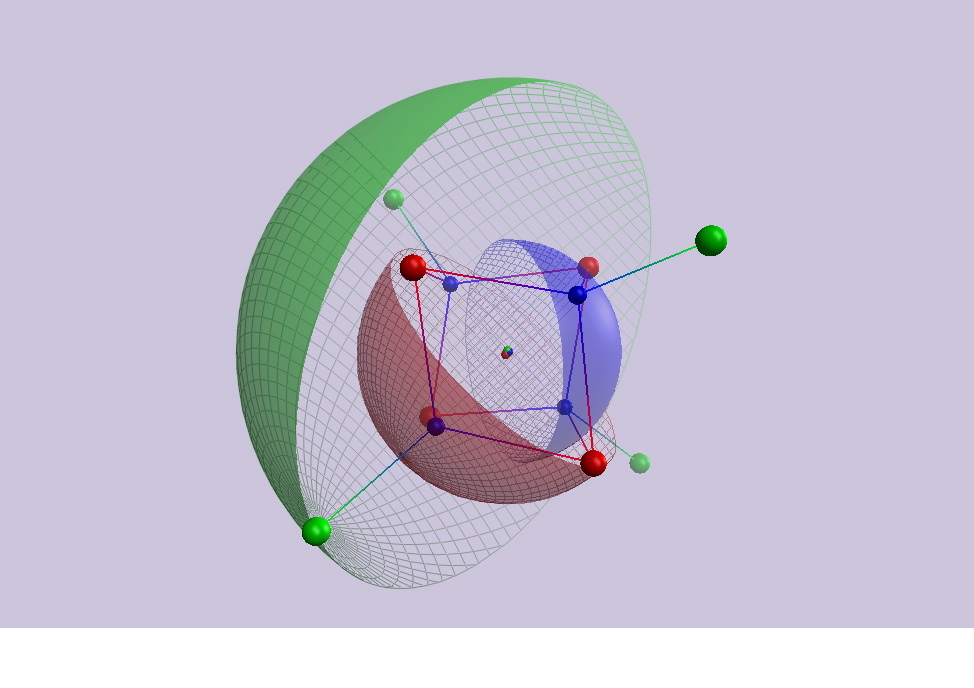
In affiliation with the Metalloprotein Structure and Design project at Scripps, the function of iron sulfur clusters in electron transfer and proton binding is being studied through a unique combination of high resolution crystal structure analysis and theoretical calculation. The detailed geometry and electrostatic properties of the iron-sulfur clusters and their protein environments are being quantitated for 7Fe ferredoxin, 2Fe Rieske and 4Fe high-potential proteins. These studies hold the promise of providing a complete description of the energetics of the oxidized and reduced states of commonly occurring types of iron-sulfur clusters in proteins, and hence a fundamental description of the basis for reduction potential and proton binding affinity.
Links:
Metalloprotein Program
Home
