

Researchers Use New Chemical Probe to Manipulate Protective Inner Barriers
By Jeff Worley, Susan Cahill, and Mika Ono
Scientists at The Scripps Research Institute and the University of California, Irvine, have developed a chemical tool that allows them to manipulate control of the passage of substances through the barriers between blood and organ tissues. The findings have important therapeutic implications for a range of conditions, including organ transplants, autoimmune disease, multiple sclerosis, and adult respiratory distress syndrome.
The research was published July 9, 2006 in an advanced online edition of Nature Chemical Biology and led by Scripps Research Professor Hugh Rosen, a principal investigator of The Scripps Research Institute Molecular Screening Center, which brings together resources from the La Jolla, California and Jupiter, Florida campuses of Scripps Research.
The group used chemical probes to gain mechanistic insight into the organization of the sphingosine 1-phosphate (S1P) system in mice, using the lung and lymphoid organs as model systems. In the study, the scientists showed they were able to switch high-affinity S1P1 receptors on and off in vivo.
The findings from this work are analogous to discovering a “wiring guide” to an extremely complex electrical system, Rosen says.
“In cases like autoimmune disease or rejection of organ transplants, or when the lungs are filling up with dangerous levels of fluid because of leaky capillaries, being able to reversibly manipulate the mechanisms that control what gets across biological barriers would be helpful to patients,” says Rosen. “This combination of probes helps us learn how to do that safely.”
Elias Zerhouni, director of the National Institutes of Health (NIH), says, “This chemical tool has implications for research on the brain, the lungs, the heart; anywhere in the body that there are biological barriers which must be crossed and whose dysfunction can result in illness,” he says. “This is exactly the kind of fundamental finding the NIH Roadmap was designed to generate, which scientists from here to academia to industry can use to accelerate their research toward practical applications.”
New Center Accelerates Research
The probe is among the first developed by scientists at the NIH-funded Scripps Research Institute Molecular Screening Center, a pilot program with equipment based in Jupiter, Florida to discover small molecule tools for translating basic biomedical discoveries more quickly into medically relevant applications.
The Scripps Research Center is one of 10 NIH-funded sites in the country dedicated to small-molecule discovery. These sites comprise a network called the Molecular Libraries Screening Centers Network, established in 2004 as part of the NIH’s strategic funding plan, the Roadmap Initiative, and guided by two NIH institutes, the National Institute of Mental Health and the National Human Genome Research Institute.
The recent milestone would have been difficult to reach, Rosen says, without the new center. Through this center, Scripps Research investigators can go from screening to optimization to “distributional tweaking,” as Rosen puts it, in order to select molecules that can work in physiological systems.
“Our key theme in this work is chemical proof-of-concept molecules with in vivo activity,” Rosen says. “What we’ve shown is that some of the barriers to making proof-of-concept small molecules in academia have now been lifted as a result of our enhanced technology, the diminished cost of screening, more highly efficient lab-scale robotics, and mass-spec pharmacokinetics. We now have the ability to define and select small molecules based upon their activities and distributional properties in animal systems, and then select for reversible perturbants of physiology and pathology.”
Proof-of-concept molecules can validate the role of particular regulatory systems in health and disease, and suggest some ways to improve approaches to therapies.
Rosen also credits the organizational structure at Scripps Research as another major enabler of success in the novel approach his team took. “To me, the key feature is one of vertical organization and integration. What we demonstrated is that multidisciplinary approaches to using integrated chemistry, biology, and high-resolution imaging are now feasible in academia and can lead to experimental insights that transform fields.”
Novel Probes
In the new study, the researchers developed probes that induced both loss of and restoration of receptor function in vivo. The researchers induced lung permeability changes by creating a matched set of chemical probes—an antagonist to make the lung leak and an agonist to reverse that damage. Rosen readily admits that this was difficult work. “Because the molecules can exist in two compositionally identical mirror-image orientations, a ‘right-handed form’ and a ‘left-handed form,’” he says, “the Wong laboratory first had to synthesize them individually and then resolve them using chiral chromatography, a process that separates handed molecules.”
Rosen explains that his group was then able to use the right-handed molecule, which is a hundred times more potent than its left-handed version, as their positive signal and the opposite-handed molecule as a negative control.
Because the S1P1 receptor is involved in the regulation of multiple physiological systems, the group was also able to track the effects of probes on the movement of lymphocytes, the cellular mediators of immunity, using multi-photon fluorescent imaging in real time in living systems.
“Though we aren’t the first to synthesize similar molecules, I believe we’re among the first to use them to figure out how to select for those that work in vivo and then to perturb biological systems in vivo to look at reversible points of physiological control,” says Rosen, who came to Scripps in 2002. “And a distinct advantage of the approach allowed us to use multi-photon fluorescent imaging to track the effects of chemical loss and restoration of function of lymphocytes in real time in a living animal.”
Rosen’s group was joined in this work by Scripps Research chemistry Professor Chi-Huey Wong, while the multi-photon imaging expertise was contributed by the groups of Michael Cahalan and Ian Parker of the University of California, Irvine.
In addition to Rosen, Wong, Cahalan, and Parker, authors of the Nature Chemical Biology study, entitled “Enhancement of Capillary Leakage and Restoration of Lymphocyte Egress by a Chiral S1P1 Antagonist In Vivo,” include: M. Germana Sanna and Sheng-Kai Wang (Scripps Research); Pedro J. Gonzalez-Cabrera ( Scripps Research and Novartis Foundation); Anthony Don (Scripps Research); David Marsolais (Scripps Research); Melanie P. Matheu (University of California, Irvine); Sindy H. Wei, (University of California, Irvine); Euging Jo (Scripps Research); and Wei-Chieh Cheng (Scripps Research).
“With a multi-talented team effort like this,” Rosen notes, “we can approach a project with many layers of depth and resolution.”
Send comments to: mikaono[at]scripps.edu
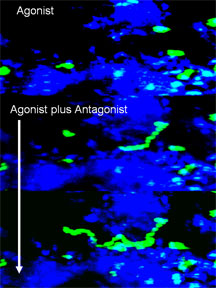
In the new study, the scientists showed they were able to switch high-affinity S1P1 receptors on and off in vivo. Click for details.
