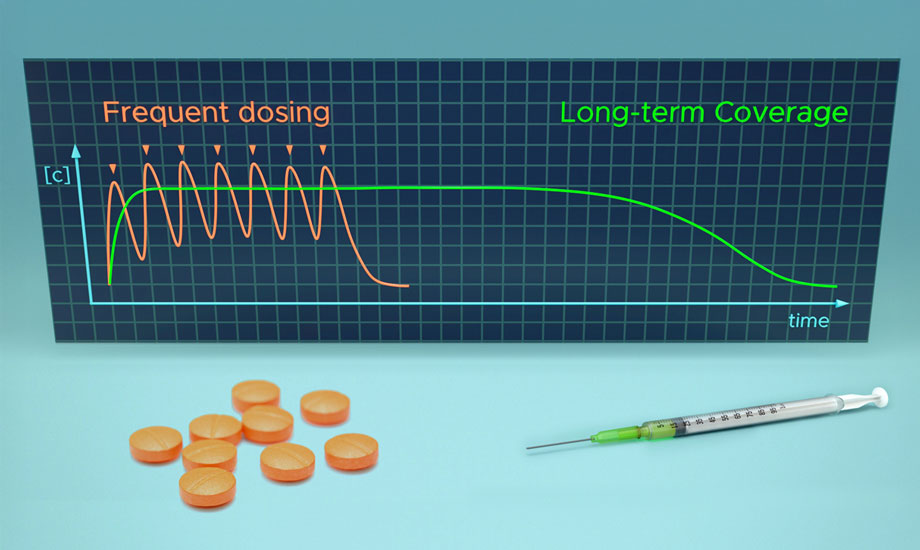
As a long-acting injectable (LAI) therapy, MMV371 is designed to improve patient compliance and provide more consistent protection against malaria.
Long-acting injectable malaria drug enters first-in-human study
Calibr-Skaggs’ long-acting injectable (LAI) platform transforms oral malaria treatment atovaquone, aiming for protection from malaria for three months.
January 23, 2025
LA JOLLA, CA—The Calibr-Skaggs Institute for Innovative Medicines, the nonprofit drug development division of Scripps Research, designed a long-acting preventative medicine for malaria (CBE161 [MMV371]) that has been administered to the first cohort of healthy volunteers in a phase 1 clinical trial. The trial is sponsored by Medicines for Malaria Venture (MMV), the leading product development partnership for malaria drug research.
Scientists at Calibr-Skaggs discovered CBE161, which was then developed as MMV371 by MMV with support from Janssen Pharmaceuticals. MMV371 is now being tested in healthy volunteers at Quotient Sciences in Nottingham, UK, with a main objective of assessing its safety, tolerability and pharmacokinetics. If successful, MMV371 clinical trials in malaria-endemic countries will begin in 2026.
Atovaquone is a well-established antimalarial agent currently administered in combination with proguanil for the prevention of malaria for travelers to endemic regions. The combination, often marketed under the trade name Malarone®, requires daily pills, and is not affordable in low- and middle-income countries.
MMV is developing MMV371 to ultimately be formulated in combination with a partner drug in line with the target product profile set for chemoprevention. As a long-acting injectable (LAI) therapy, it is designed to improve patient compliance and provide more consistent protection against malaria. Such an innovation would have a profound impact in regions with high malaria transmission.
The Calibr-Skaggs team selected atovaquone for LAI optimization since it is an approved malaria drug with favorable safety, tolerability and chemical properties. MMV371, if successful, represents a significant step forward in the fight against malaria, as it aims to meet the MMV target product profile of providing at least three months of protection with a single injection.
“Our LAI platform transforms existing therapies into long-acting injectables, significantly improving patient compliance and extending protection,” says Anil Gupta, director of medicinal chemistry at Calibr-Skaggs and a lead chemist on the discovery of CBE161. “These long-acting therapies require less drug to be effective, reducing waste and cost, which is especially important in resource-limited areas.”
To create the long-acting atovaquone analog CBE161, Gupta and his team tried several ways of extending atovaquone’s time in circulation. As described in a recent preprint, they landed on a prodrug approach: This involves attaching a chemical group to the drug, which after injection is then removed by chemical or enzymatic processes in the body to reactivate the drug. They created several different prodrugs and tried various ways of delivering them. In an animal model, intramuscular (IM) injection of CBE161 maintained atovaquone levels above the minimal effective concentration for 69 days compared to 21 days with IM injection of atovaquone alone. These data indicate that CBE161 should extend the life of active atovaquone in the bloodstream of humans, enhancing its effectiveness with fewer injections.
For people in malaria-endemic regions, current chemoprevention therapies for malaria have strict, frequent dosing regimens that are difficult to adhere to—providing opportunities for chemoresistance to evolve when not followed. MMV371 shows promise in extending protection with fewer doses and may also combat malaria resistance with a simpler administration schedule. Additionally, fewer doses could potentially reduce costs and enhance global malaria control efforts, particularly in high-prevalence, limited healthcare regions.
“Because the LAI platform can expand access to effective therapies, we have the opportunity to leverage LAIs to treat multiple diseases and improve global health with lower-cost medicines that are easier to administer and meet patient needs,” says Arnab Chatterjee, vice president of Medicinal Chemistry at Calibr-Skaggs. “This is an exciting time for the LAI team at Calibr-Skaggs, as we have LAIs undergoing phase 1 clinical trials for both malaria and HIV.”
About Scripps Research
Scripps Research is an independent, nonprofit biomedical institute ranked one of the most influential in the world for its impact on innovation by Nature Index. We are advancing human health through profound discoveries that address pressing medical concerns around the globe. Our drug discovery and development division, Calibr-Skaggs, works hand-in-hand with scientists across disciplines to bring new medicines to patients as quickly and efficiently as possible, while teams at Scripps Research Translational Institute harness genomics, digital medicine and cutting-edge informatics to understand individual health and render more effective healthcare. Scripps Research also trains the next generation of leading scientists at our Skaggs Graduate School, consistently named among the top 10 US programs for chemistry and biological sciences. Learn more at www.scripps.edu.
About the Calibr-Skaggs Institute for Innovative Medicines
The Calibr-Skaggs Institute for Innovative Medicines was founded on the principle that the creation of new medicines can be accelerated by pairing world-class biomedical research with state-of-the-art drug discovery and development capabilities. Leveraging the unique scientific framework of Scripps Research, Calibr-Skaggs has created a portfolio of drug candidates based on Scripps Research technologies and is shaping a new paradigm for advancing nonprofit biomedical research to impact patients. Learn more at calibr.scripps.edu.
About Medicines for Malaria Venture (MMV)
MMV is a Swiss-based not-for-profit organization working to deliver a portfolio of accessible medicines with the power to treat, prevent and eliminate malaria. Born in 1999, out of a need for greater health equity, we close critical gaps in research, development and access – working “end-to-end” to expand the use of existing antimalarials and innovate new compounds to protect public health. This starts with women and children.
It’s working. As of 2023, MMV-supported products have effectively treated an estimated 680 million people and saved around 15.4 million lives. We cannot stop now.
With a quarter of a billion malaria cases and more than 600,000 deaths reported in 2022, progress towards disease elimination has stalled. MMV is part of an ecosystem of partners determined to change this. Bringing public and private sector partners together, we pioneer new solutions that align with local and global health priorities and promote the equitable development of effective and affordable products that work to help end malaria and advance health for all.
For more information, visit http://www.mmv.org.
For more information, contact press@scripps.edu

