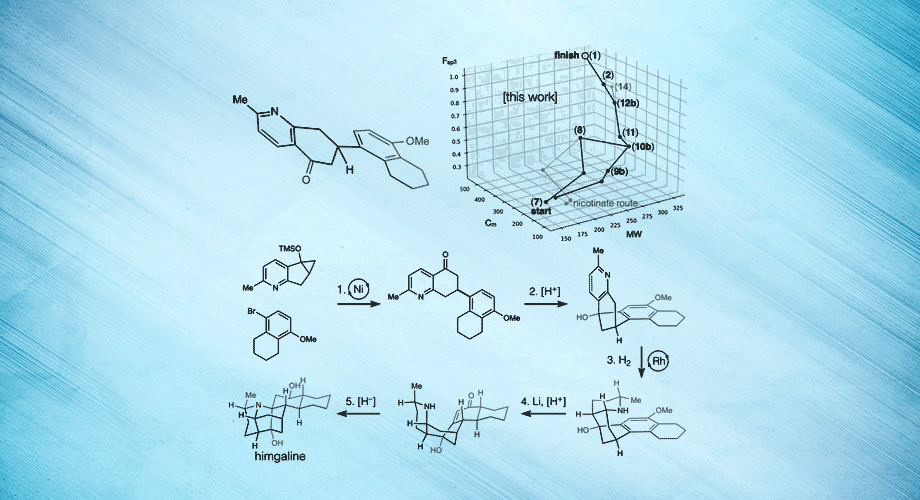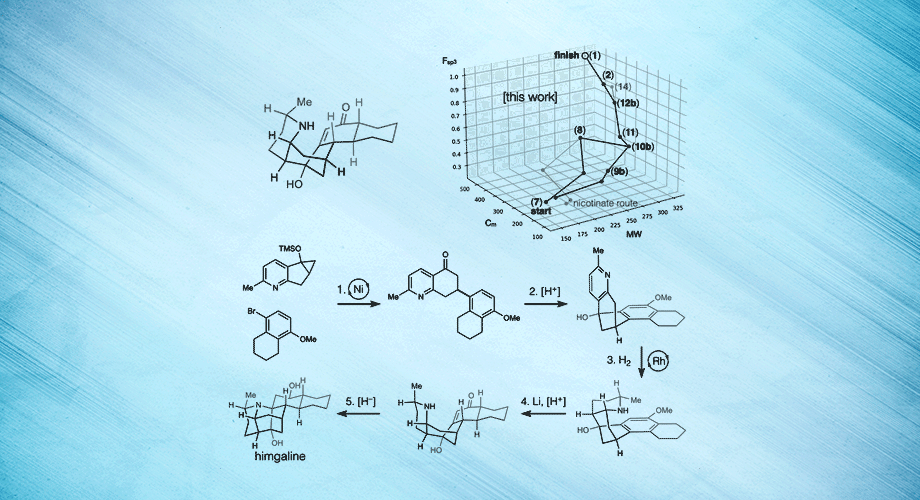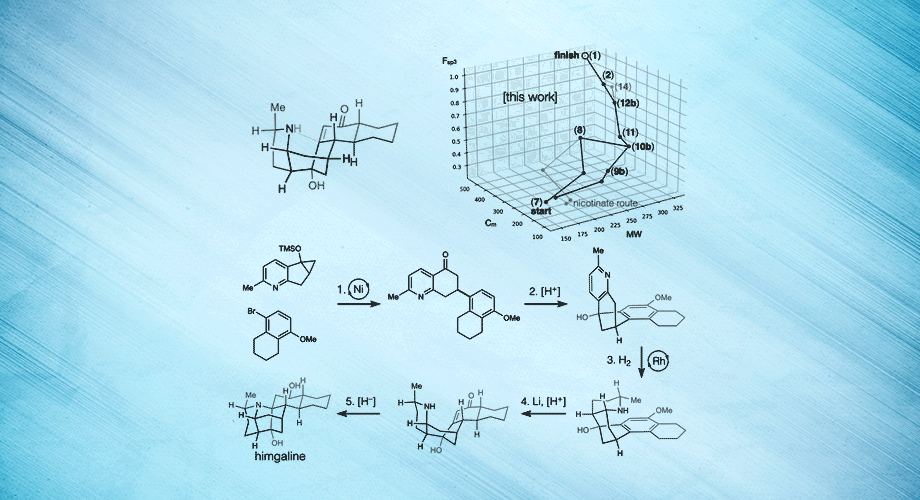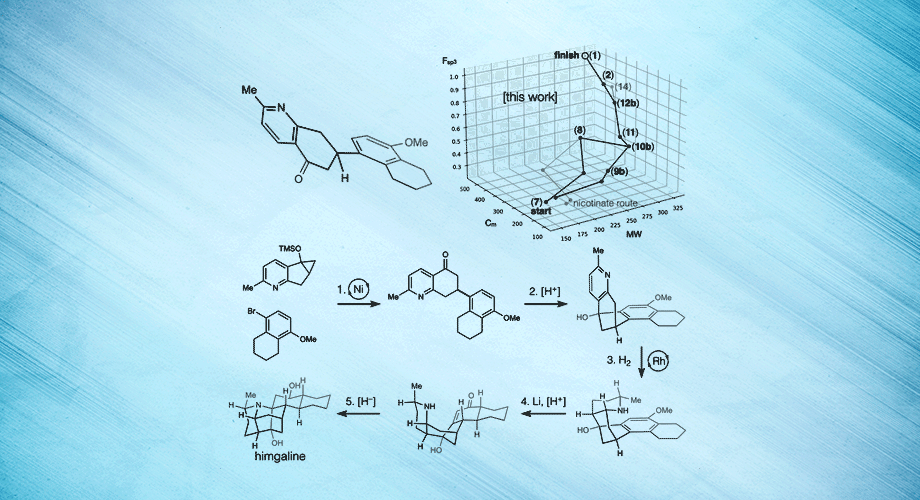
Three views of a breakthrough chemical synthesis of the neuroactive plant metabolite himgaline. Hydrogen atoms are used to fold flat molecules into specific, three-dimensional shapes. Credit: Scripps Research.
Scripps Research chemists find a quick way to synthesize novel neuroactive compounds found in rainforest tree
Bark of Galbulimima belgraveana contains compounds that could be the basis for new psychiatric and neurological drugs.
March 16, 2022
LA JOLLA, CA—A potential cornucopia of neuroactive compounds, which might yield clues to the design of future psychiatric and neurological drugs, has become more accessible to synthetic chemists, thanks to new work from Scripps Research.

Scripps Research chemists discovered a quick way to synthesize novel neuroactive compounds found in the bark of the Galbulimima belgraveana tree, which could be the basis for new psychiatric and neurological drugs. Credit: CSIRO.
The discovery, reported March 17, 2022, in Science, concerns compounds contained in the rainforest tree Galbulimima belgraveana and its close cousin Galbulimima baccata, which are native to Papua New Guinea, tropical northern Australia, and Malaysia.
Potions made from the bark of these trees have long been known to have hallucinogenic and other neuroactive effects, but the precise compounds involved, and their biological targets, have largely been a mystery. The Scripps Research chemists found what is essentially the first streamlined, practical method for synthesizing many of these compounds.
“We’re very interested in learning how these Galbulimima compounds affect the brain, and hope to derive useful new therapeutics from them. Now with this improved approach to making these molecules, we can start to do just that,” says Ryan Shenvi, PhD, the professor of chemistry at Scripps Research who led the study.
The co-first authors of the study were Eleanor Landwehr, Meghan Baker PhD, and Takuya Oguma PhD, who worked in the Shenvi lab during the study.
The neuroactive effects of compounds found in Galbulimima bark were first highlighted just after World War II in surveys by the pharma company Smith, Kline & French and the Australian national research organization CSIRO. Until now, though, the dense structural complexity of these compounds, their variable mix within Galbulimima bark, and the difficulty of obtaining that bark in quantity, have prevented their close study. Indeed, chemists had devised a concise and practical synthesis for only one of these compounds, himbacine.
In the new study, Shenvi and his team targeted another Galbulimima compound called himgaline, which, like himbacine, appears to have antispasmodic properties, though it is likely to act in a different way. Whereas the best prior method to synthesize himgaline requires 19 steps—too many for routine use—the new method took only 7 steps, enabling easy synthesis at the scale needed to study the compound in detail.
Shenvi says the new method is more efficient in part because it starts with a broad approach to the chemical “space” or “neighborhood” around himgaline, allowing that specific compound—or other related compounds—to be made relatively easily from there. Using this approach, the team demonstrated syntheses of himgaline and two other Galbulimima compounds, GB22 and GB13.
“Our approach is somewhat analogous to distant space travel—we first try to get to the target star system, so to speak, and from there it’s relatively easy to get to specific planets within that system,” Shenvi says.
Shenvi and his team are now following up with studies of the biological properties of himgaline, GB22 and GB13, and are also using their broad synthesis strategy to make and study other Galbulimima compounds.
“Concise syntheses of GB22, GB13, and himgaline by cross-coupling and complete reduction” was co-authored by Eleanor Landwehr, Meghan Baker, Takuya Oguma, Hannah Burdge, Takahiro Kawajiri and Ryan Shenvi, who were all at Scripps Research during the study.
The work was funded in part by the National Institutes of Health (GM122606) and the National Science Foundation (CHE1856747).
For more information, contact press@scripps.edu

