
An electron microscopy image of lymph node tissue from a mouse that was immunized with an experimental COVID-19 vaccine. Vaccine particles are readily taken up by immune cells, stimulating antibody production for several weeks. (Image courtesy of Yi-Nan Zhang, Kimberly Vanderpool, Theresa Fassel and Scott Henderson)
Experimental COVID-19 vaccine appears to work well against variants of concern
Results from studies in mice suggest the protein-based vaccine could potentially serve as a booster shot to cover emerging COVID-19 variants.
March 29, 2021
LA JOLLA, CA—An experimental COVID-19 vaccine that uses virus-shaped particles to stimulate an immune response performs well against the initial strain of the virus as well as three major “variants of concern,” according to an early-stage study in mice from scientists at Scripps Research.
The results, which need to be confirmed in clinical trials, suggest the vaccine could be useful in protecting against new SARS-CoV-2 variants as they arise, says Jiang Zhu, PhD, associate professor in the Department of Integrative Structural and Computational Biology at Scripps Research and inventor of the vaccine.
The three existing variants of concern—known as B.1.1.7, B.1.351 and P.1—have spread widely in recent months, especially in the UK, South Africa, Brazil and Europe. Preliminary evidence suggests that some or all of them may be less susceptible to the COVID-19 vaccines now in use.
The new study, which appears in the online preprint journal BioRxiv , also includes an imaging-based analysis of the immune response in mice following their inoculation with the new vaccine.
“The effectiveness of our vaccine against new variants in this preclinical study is exciting, as are some of the ‘firsts’ we achieved in vaccine immunology,” says Zhu, who led the study. “For example, we were able to directly image our vaccine particles in the lymph node tissues of inoculated mice, capturing the start of the antibody response.”
The vaccine design presents the SARS-CoV-2 “spike” protein, the key target of the immune response, on the surface of spherical protein particles, mimicking the shape and size of the actual virus. By contrast, most of the current COVID-19 vaccines present or cause the body’s cells to produce only the spike. In a preclinical study published in Science Advances on March 19, Zhu and colleagues found evidence that their whole-virus-mimicking approach stimulated a stronger immune response than spikes alone.
The team confirmed those findings in their latest research, conducting new experiments with different vaccine dosages and injection routes. They showed that their vaccine induces an antibody response in mice that strongly neutralizes the three variants of concern: B.1.1.7, B.1.351, and P.1. In fact, Zhu says, it neutralizes them just as strongly as it neutralizes the original reference strain of the virus, known as Wuhan-Hu-1.
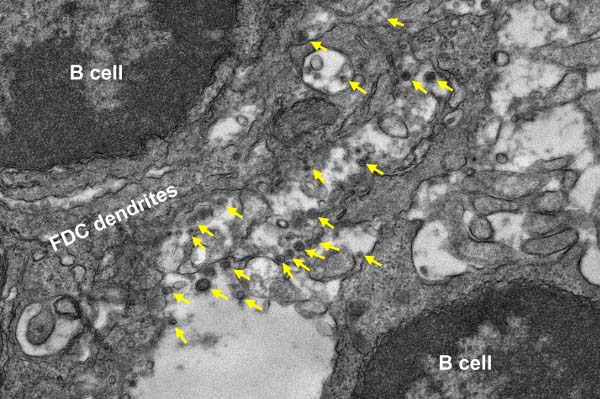
An electron microscopy image of lymph node tissue from a mouse immunized with the experimental COVID-19 vaccine. Arrows point to protein nanoparticles that are aligned on dendrites of a follicular dendritic cell (FDC) and interact with surrounding B cells. (Image courtesy of Yi-Nan Zhang, Kimberly Vanderpool, Theresa Fassel and Scott Henderson.)
In their previous study, Zhu and colleagues also found that their experimental vaccine seems to work well also against SARS-CoV-1, a more distantly related coronavirus that caused deadly outbreaks chiefly in Asia in 2002 to 2004. Thus, Zhu says, the new vaccine so far appears to induce unusually broad protection, potentially making it well suited for use against an ever-evolving SARS-CoV-2.
For the new study, Zhu and his team performed microscopic analyses of the lymph nodes of inoculated mice. Lymph nodes are among the key places in the body where immune cells normally encounter circulating viruses—or viral proteins from vaccines—and initiate a long-term antibody response.
The scientists showed, with striking transmission electron microscopy images, that their virus-mimicking vaccine particles are readily taken up, intact, by immune cells in mouse lymph nodes. They also observed that the particles remain in place, stimulating the B cells that produce antibodies, for several weeks—many times longer than free-floating spike proteins.
“Having larger, more virus-like particles, as our vaccine does, appears to make a big difference in terms of stimulating a stronger and broader response,” says Yi-Nan Zhang, PhD, a postdoctoral researcher in Zhu’s lab who was first author of the study.
The researchers hope to test their approach soon in clinical trials.
The study, “Mechanism of a COVID-19 nanoparticle vaccine candidate that elicits a broadly neutralizing antibody response to SARS-CoV-2 variants” was authored by Yi-Nan Zhang, Jennifer Paynter, Tatiana Fourfouris, Cindy Sou, Timothy Ngo, Linling He and Jiang Zhu.
Support for the work was provided by grants from the National Institutes of Health (AI137472, AI139092) and by Ufovax Bio LLC, a vaccine development company co-founded by Zhu.
For more information, contact press@scripps.edu

